Case Report
A 44-year-old woman presented at the Surgical Outpatient Department with anterior neck swelling of 4-year duration measuring 9 cm (vertical) ¥ 13 cm (transverse). The swelling was bigger to the right of midline than to the left and her trachea was deviated to the left. However, she had neither pressure symptoms nor any other symptoms. Clinical diagnosis of simple multinodular goiter was made and thyroid function tests done showed normal parameters. Her hematocrit was 34%. She was offered subtotal thyroidectomy using blood-conservation techniques and she consented. A blood- conservation plan was drawn up and anesthesiologist’s review sought. From the first outpatient visit the patient was placed on oral ferrous gluconate 900 mg (105 mg elemental iron) daily along with adjuncts (Vitamin C, Vitamin B Complex, and Multivitamins). Six weeks later the patient’s hematocrit was 40.5%, and the patient was then booked for surgery 5 days later. In theatre, acute normovolemic hemodilution (ANH) was done before induction of general endotracheal anesthesia, during which one unit of whole blood was withdrawn and replaced with 1500 mL of normal saline. The patient was positioned in Reverse Trendelenburg and theatre temperature was maintained above 27°C. Non-invasive monitoring with pulse oximeter was used throughout surgery. Intraoperatively careful hemostasis was ensured with diathermy and ligatures, and the thyroid vessels were secured early (see Figure 1). Hemostats were care- fully placed around the thyroid capsule before the left and right lobes of the gland were partially excised leaving about 8 g of thyroid tissue and the parathyroid glands (see Figure 2). The patient’s one unit of blood which was withdrawn during ANH was re-infused while closing up. Estimated blood loss at the end of surgery was 300 mL. The patient made uneventful recovery and remained on oral hematinics postoperatively. She was discharged on the third postoperative day with hematocrit of 41%. Repeat hematocrit 3 weeks postoperatively was 40.6% and the patient remained well without any complaint (Figure 3).
Figure 1. Securing the thyroid vessels early by ligation before gland excision.
Figure 2. Right (larger) and left lobes of thyroid gland excised with minimal blood loss.
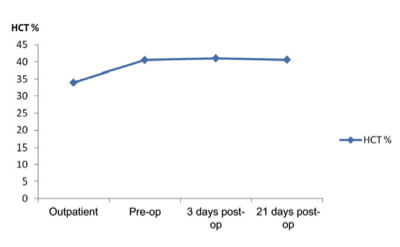
Figure 3. Rise in patient’s hematocrit (HCT) on oral hematinics.
Discussion
Blood conservation involves combining various techniques individualized to the patient in order to avoid the use of allogeneic blood.¹ It was introduced initially as ‘Bloodless Surgery’ for the care of patients who refused blood transfusion, notably Jehovah’s Witnesses.² From being a novelty practiced by few surgeons, blood conservation has now become widely regarded as the standard of care on account of adverse outcome reported with blood transfusion in all surgical subspecialties and, conversely, improved outcome with transfusion restricted protocols.³ ⁴ Thus, it is now sometimes physician-initiated, with the aim of improving outcome through evidence-based practice, even for non Jehovah’s Witness patients, as in this case.⁵
Thyroidectomy is historically associated with much blood loss, though knowledge of anatomy and developments in surgical hemostasis made it safer in the last century.⁶ A fall in the hematocrit is thus to be expected postoperatively, for which allogeneic blood is sometimes employed perioperatively in > 2–5% of cases in good centers and somewhat more in others.⁷ In the index patient, however, a rise in hematocrit rather than a fall was recorded postoperatively as a result of employing blood-conservation techniques.
The three basic principles of blood conservation may be summarized as follows: (i) optimizing the hematocrit; (ii) minimizing blood loss; and (iii) lowering the transfusion trigger.³ To these may be added a fourth: ‘Optimizing oxygen delivery’.² Various techniques have been developed using these principles to care for surgical patients without the use of allogeneic blood. The effectiveness of these techniques in reducing morbidity and mortality and length of hospital stay when used in a patient-specific combination is well established.² Two major principles of blood conservation that were relevant to our patient are discussed here.
| Hematological parameters | Initial outpatient visit | Preoperative (after 6 weeks’ hematinics) | 3 days postoperative | 21 days postoperative |
| Hematocrit (%) | 34 | 40.5 | 41 | 40.6 |
| Hemoglobin (g/dL) | 11.3 | 13.5 | – | 13.7 |
| Mean corpuscular volume (fL) | – | 88.6 | – | 89.8 |
| Mean corpuscular hemoglobin (pg) | – | 29.7 | – | 30.3 |
| Mean corpuscular hemoglobin
concentration (g/dL) |
– | 33.5 | – | 33.7 |
Optimizing the hematocrit
Raising the hematocrit preoperatively increases the margin of safety in the event of blood loss. The index patient had anemia WHO Grade 0 (hemoglobin ? 11 g/dL) in the outpatient department, and iron studies in our resource-poor setting were out of the question (Table 1).⁸ ⁹ Oral iron at prophylactic dosage along with Vitamin C, Vitamin B Complex and Multivitamins, was started on this patient on the first outpatient visit and proved adequate to raise the hematocrit from 34% to 40.5% in 6 weeks, confirming the effectiveness of oral iron in stimulating erythropoiesis.¹⁰ Intravenous iron is rarely necessary in treating preoperative anemia. Surgery was not deliberately delayed in order to raise the hematocrit, but while waiting for the patient to be fully investigated, to organize herself for surgery and take her turn on the operation register, she was maintained on oral hematinics. The erythrogenic effect of the hematinics obviously persisted through surgery into the postoperative period, and resulted in a hematocrit of 41% on the third postoperative day.
Minimizing blood loss
ANH dilutes the patient’s blood and reduces the red blood cell mass loss intraoperatively.¹² Reconstitution at the end of surgery with the patient’s undiluted blood obviously helps to restore the hematocrit. It was possible to withdraw as much four units safely but we chose to withdraw one, and this was before induction of anesthesia because we did not have sophisticated monitoring. We used crystalloids in replacement rather than colloids to achieve greater hemodilution. Positioning the patient with the operation site above the right atrium reduces hemorrhage from incised vessels and general oozing from the op site.¹ ¹² Normothermia averts coagulopathy that could occur with hypothermia.¹ ² Noninvasive monitoring is also known to limit intraoperative blood loss. Meticulous hemostasis combined with other techniques mentioned above resulted in the estimated 300 mL of dilute blood lost intraoperatively which had a minimal effect on the patient’s hematocrit. Although we had a topical glue (Surgicel®, Johnson & Johnson, Somerville, NJ, USA) on hand, it was never used. Careful and pre-emptive ligation of vessels, following the principles of thyroid surgery, and use of diathermy ensured adequate hemostasis.
This case report demonstrates that it is possible to maintain a normal hematocrit throughout the perioperative period in high-risk major surgery by using blood-conservation techniques. The erythrogenic effect of oral hematinics can be quite significant as in this case. Early prophylactic hematinics should be considered whenever possible and maintained through the perioperative period. On its own, the benefit of ANH in this patient may have been small, but when added to the effects of other techniques used, the overall benefit becomes significant. Thus, patient-specific combination of techniques and a multidisciplinary approach enhances the efficacy of blood conservation. The outcome in this case makes a strong case for the effectiveness of bloodless surgery. Moreover, avoiding allogeneic blood transfusion in a community setting where HIV has a high prevalence and screening of blood may be suboptimal is certainly to be recommended, and patient blood management as demonstrated in this case report is the ideal clinical approach.
Acknowledgments
- Seeber P, Shander A. Basics of Blood Management. Blackwell Publishing: New York, 2007.
- Martyn V, Farmer SL, Wren MN, et al. The theory and practice of bloodless surgery. Transfus Apher Sci 2002; 27: 29–43.
- Spahn DR, Moch H, Hofmann A, Isbister JP. Patient blood management: the pragmatic solution for the problems with blood transfusions. Anesthesiology 2008; 109: 951–3.
- Marik PE, Corwin HL. Efficacy of red cell transfusion in the critically ill: a system- atic review of the literature. Crit Care Med 2008; 36: 2667–74.
- Boucher BA, Hannon TJ. Blood management: a primer for clinicians. Pharmaco- therapy 2007; 27: 1394–411.
- Lyerly HK. The thyroid gland – historical aspects and anatomy. In: Sabiston DC (ed.). Textbook of Surgery: the Biological Basis of Modern Surgical Practice, 14th edn. W. B. Saunders: Philadelphia, PA, 1991, pp. 556–60.
- Pieracci FM, Fahey TJ. Effect of hospital volume of thyroidectomies on outcomes following substernal thyroidectomy. World J Surg 2008; 32: 740–6.
- Groopman JE, Itri LM. Chemotherapy- induced anemia in adults: incidence and treatment. J Natl Cancer Inst 1999; 91: 1616–34.
- World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention and Control – A Guide for Programme Managers. World Health Organization: Geneva, 2001.
- Beris P. The use of iron to increase red cell mass. Can J Anesth 2003; 50: S3–S9.
- Beris P, Muñoz M, García-Erce JA, et al. Perioperative anaemia management: consensus statement on the role of intra- venous iron. Br J Anaesth 2008; 100: 599–604.
- Goodnough LT, Shander A, Spence R. Bloodless medicine: clinical care without allogeneic blood transfusion. Transfusion 2003; 43: 668–76.